Chemistry: What Are Acids and Bases?
What Are Acids and Bases?
Although I've told you that acids and bases aren't hard to understand, I've got bad news: There are not one but three common definitions used to describe acids and bases: Arrhenius acids and bases, Brnsted-Lowry acids and bases, and Lewis acids and bases. Though this makes it sound as if you'll have to learn about acids and bases three times, the good news is that for many practical purposes, these three definitions are roughly equivalent.
Arrhenius Acids and Bases
Way back in the late 1800s, our old friend Svante Arrhenius came up with definitions of acids and bases while working on kinetics problems.
According to Arrhenius, acids are compounds that break up in water to give off hydronium (H+) ions. A common example of an Arrhenius acid is hydrochloric acid (HCl):
- HCl ⇔ H+ + Cl-
The formulas for acids usually start with hydrogen, though organic acids are a notable exception. The names and formulas of some common acids are given in the table below:
| Acid Name | Formula |
|---|---|
| hydrochloric acid | HCl |
| nitric acid | HNO3 |
| phosphoric acid | H3PO4 |
| sulfuric acid | H2SO4 |
| acetic acid | C2H4O2 |
Arrhenius bases are defined as compounds that cause the formation of the hydroxide ion when placed in water. One example of an Arrhenius base is sodium hydroxide (NaOH):
- NaOH ⇔ Na+ + OH-
Bases typically have "OH" in their formulas, though there are exceptions. For example, ammonia (NH3) doesn't contain hydroxide ions but forms them when it reacts with water:
- NH3 + H2O ⇔ NH4+ + OH-
The names and formulas of some common bases are in the following table:
| Base Name | Formula |
|---|---|
| ammonia | NH3 |
| potassium hydroxide | KOH |
| sodium bicarbonate | NaHCO3 |
| sodium carbonate | Na2CO3 |
| sodium hydroxide | NaOH |
Some oxides form acids or bases when water is added. Because these compounds don't contain any H+ or OH- ions unless they react with water, they're called "anhydrides." Typically, oxides of nonmetals are acid anhydrides (they form acid when placed in water), and oxides of metals are base anhydrides (forming a base when placed in water).
Brnsted-Lowry Acids and Bases
In the early 1900s, an alternate definition for acids and bases was proposed by Johannes Brnsted and Thomas Lowry to account for the fact that ammonia can neutralize the acidity of HCl even if water isn't present. This phenomenon showed them that ammonia is a base, even when there isn't water around to form hydroxide ions.
The Mole Says
There are many different names and formulas used to describe the hydronium ion. Though the formula was shown previously as "H+", it is sometimes written as "H3O" because this is the ion formed when H+ combines with water. Another common way of referring to hydronium ions is just to call them "protons." This name comes from the fact that H+ represents a hydrogen atom (one proton and one electron) that has lost its electron, leaving only the bare proton behind.
A Brnsted-Lowry acid is defined as a compound that gives hydronium ions to another compound—for example, hydrochloric acid gives H+ ions to compounds it reacts with. Brnsted-Lowry bases are compounds that can accept hydronium ions—when ammonia gets a hydronium ion from HCl, it forms the ammonium ion.
The following equation represents the reaction of a Brnsted-Lowry acid with a Brnsted-Lowry base:
- HNO3 + NH3 ⇔ NO3- + NH4+
In this reaction, nitric acid behaves as an acid because it gives a proton to ammonia. Ammonia behaves as a base because it accepts the proton from nitric acid.
However, if you take a look at the other side of the equation, we find the nitrate and ammonium ions. Because the nitrate ion can accept protons from the ammonium ion (to form HNO3), the nitrate ion is a very weak Brnsted-Lowry base. Because the ammonium ion has an extra proton to donate (in this case to the nitrate ion), it is a Brnsted-Lowry acid.
The nitrate ion is based on the nitric acid molecule, so we say that it is the conjugate base of nitric acid. Likewise, the ammonium ion is the conjugate acid of ammonia. Together, an acid with its conjugate base (such as HNO3 and NO3-) or a base with its conjugate acid (such as NH3 and NH4+) is referred to as a conjugate acid-base pair.
Lewis Acids and Bases
In the Brnsted-Lowry definition of acids and bases, a base is defined as a compound that can accept a proton. However, how does it accept the proton?
One feature that Brnsted-Lowry bases have in common with each other is that they have an unshared pair of electrons. When a hydronium ion comes wandering by the molecule, sometimes the lone pairs will reach out and grab it. An example of this is when ammonia accepts a proton in an acidic solution:
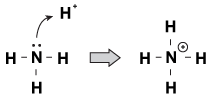
Figure 23.1Ammonia can grab a proton from nitric acid with its lone pair of electrons.
One way of looking at this process is that the ammonia atom is donating its lone pair to the proton. Because the lone pairs are driving this chemical reaction, we have a new definition of acidity and basicity, called "Lewis acidity/basicity." A Lewis base is a compound that donates an electron pair to another compound (the ammonia in our example). A Lewis acid is a compound that accepts an electron pair (the H+ ion in our example).
Molecular Meanings
Lewis bases are chemicals that can donate electron pairs. Lewis acids are chemicals that can accept them.
Though we had ammonia donating a lone pair to a proton in our example, the lone pair in ammonia can react with a lot of other compounds as well. For example, ammonia can donate its lone pair of electrons to BH3 by the following process:

Figure 23.2The lone pair on ammonia attaching itself to BH3.
In this process, ammonia is the Lewis base and BH3 is the Lewis acid.
Generally, the Lewis definition of acids and bases is the most useful because it is the most inclusive of the three definitions. For example, the Brnsted-Lowry definition of an acid includes HF but not BH3, which doesn't lose a proton when attached by the lone pairs on a Lewis base.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
