DK Science: Water
The simple combination of two hydrogen atoms and one oxygen atom creates a water molecule (H2O). Water is the most common compound on Earth, making up over half the weight of living things. It is vital to life, bringing nutrients to and taking away waste from every living cell. Water molecules are attracted to one another through HYDROGEN BONDS, and this gives water some unusual but useful properties.

More substances dissolve in water than in any other liquid. Its molecules are small and have a slight electrical charge, so they can move around and interact with other particles. If water did not have this property, life could not exist. Water is nature’s carrier. Dissolved gases, such as oxygen and carbon dioxide, are carried by water to and from all living cells.
Water covers around 70 per cent of the Earth’s surface. This is why Earth looks blue from space, and why it is often called the Blue Planet. Water is liquid in the oceans and forms solid ice caps at the ice caps. Water vapour is a gas in air. Humid places, such as rainforests, have a lot of water vapour. The human body is about 60 per cent water and a ripe tomato contains over 95 per cent water.
At room temperature, pure water is a colourless liquid with a neutral pH – it is not an acid or a base. But most water is not pure. Hard water contains calcium and magnesium minerals, which have dissolved in the water as it flows over rocks. Soap does not lather well in hard water – the minerals react with the soap to form a scum. Hard water is softened by boiling or by passing it through a water softener.
Water molecules have an attraction to other water molecules. This attraction is called the hydrogen bond. It is a fairly weak bond compared to the bonds within a water molecule, but it is still strong enough to give water some unusual properties. For example, water is a liquid at room temperature; other molecules of a similar size are gases. It is also less dense as a solid than as a liquid.
In a water molecule, electrons are pulled closer to the oxygen atom than the hydrogen atoms. So the oxygen atom has a small negative charge, and the hydrogen atoms have a small positive charge. The slightly positively charged hydrogen atoms of one water molecule are attracted to the slightly negatively charged oxygen atoms of another water molecule. This attraction is the hydrogen bond.
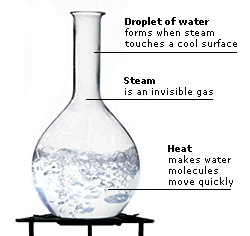
Water boils at 100ºC (212ºF). This is almost 200ºC (424ºF) higher than the boiling points of other similar-sized molecules, such as hydrogen sulphide. Water’s high boiling point can be explained by its hydrogen bonds. Extra heat is needed to break the hydrogen bonds, so a water molecule can break free of other water molecules and leave the liquid’s surface as steam, which is a gas.
