Chemistry: Converting Between Moles, Molecules, and Grams
Converting Between Moles, Molecules, and Grams
Once you know how to find molar mass, you can start to convert between moles, grams, and molecules of a substance. To do so, use the following figure:
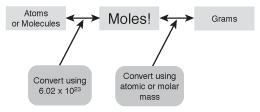
Figure 11.2By using this map as a guide, you can easily learn to convert between grams, molecules, and moles of chemical compounds.
The method you'll use to solve this problem is the factor-label method. We'll see how to use the factor label method using the following example:
Example: Convert 63 grams of ammonia to molecules.
Solution: To answer this problem, do the following steps:
- Write down the number you're trying to convert.
63 g NH3 - Write a multiplication sign after the unit you're trying to convert, followed by a straight, horizontal line.
63 g NH3 × ________________ - In the space below the line, write the unit of the number you're trying to convert.
63 g NH3 × ________________⁄ g NH3 - Take a look at the map in the preceding figure. Find your starting point (in this case, "g") and move one box over toward your destination. Write the unit that you find in the next box (in this case, "mol") on top of the line.
63 g NH3 × mol NH3⁄g NH3 - Write the conversion factor in front of the units you wrote above and below the line. This conversion factor can be found on the line between the starting and destination boxes in the figure (in this case, it's the molar mass of NH3).
63 g NH3 × 1 mol NH3⁄17 g NH3 - Multiply these numbers together, making sure to cancel out any appropriate units.
63 g NH3 × 1 mol NH3⁄17 g NH3 = 3.7 mol NH3 - If you haven't yet finished the problem, use the output from your first calculation to start a second calculation using steps 1-6.
Bad Reactions
When using this method, always write "1" in front of the word "moles," "6.02 × 1023" in front of the unit "atoms" or "molecules," and the molar mass in front of the unit "grams." If you don't, your answer will be wrong!
You've Got Problems
Problem 2: What is the mass of 4.3 × 1022 molecules of POCl3?
In this case, we found that we have 3.7 moles of NH3. However, we're trying to find the number of molecules of ammonia, not the number of moles. As a result, we need to go through the preceding six steps to find molecules. When this calculation is set up, it should look like this:
- 3.7 mol NH3 × 6.02 × 1023 molecules NH3⁄1 mol NH3 molecules NH3 = 2.2 × 1024 molecules
Our answer, then, is 2.2 × 1024 molecules NH3.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
