Chemistry: Gas Stoichiometry
Gas Stoichiometry
Though we've limited our discussion of stoichiometry to grams and moles, we can also do stoichiometric calculations for gases using volume. However, in order to do this, we need to modify our diagram slightly:
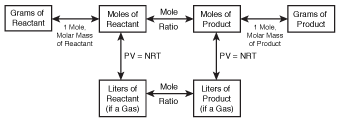
Figure 19.3Our stoichiometric diagram, modified to include gases.
In order to use this diagram, we need to be able to convert from liters of a gas to moles. Fortunately, we learned how to do this in Gas Laws with the ideal gas law. If you've forgotten how to use the ideal gas law, it might be a good idea to brush up on it before continuing with this section!
Aside from this change, stoichiometric calculations for gases are done in exactly the same way. Start at the box that includes the information you've been given, and move through the diagram, box by box, until you arrive at your desired destination. Let's do an example:
Example: For the reaction 2 H2(g) + O2(g) ⇔ H2O(g), determine how many liters of hydrogen gas will be required to produce 175 grams of water vapor (steam). Assume that you have an excess of oxygen gas, a partial pressure of hydrogen of 1.00 atm, and a temperature of 20º C.
Solution: This problem is solved in exactly the same way as the other stoichiometry problems in this section. In order, we'll need to convert the number of grams of steam to moles of water, then moles of water to moles of hydrogen, and finally the moles of hydrogen to liters of hydrogen.
Step 1
Convert grams of steam to moles of water:
- 175 g H2O × 1_mol_H2O⁄18.0 g H2O
Step 2
Convert moles of water to moles of hydrogen:
- 175 g H2O × 1 mol H2O⁄18.0 g H2O × _2_mol_H2_⁄2 mol H2O = 9.72 mol H2
Step 3
Convert moles of hydrogen to liters of hydrogen using the ideal gas law, PV=nRT. In this example, P = 1.00 atm, V is unknown, n = 9.72 mol, R = 0.08206 L atm/mol K, and T = 293 K (remember always to convert degrees Celsius to Kelvin when doing gas law problems):
- (1.00atm)(V) = (9.72mol)(0.08206Latm⁄molK)(293K)
- V = 234 L

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
