Chemistry: Intermolecular Forces
Intermolecular Forces
You may be familiar with the "ball pits" that can frequently be found in the play areas at fast food restaurants to keep little kids amused. They contain thousands of hollow plastic balls in a big pit that kids can jump around in and throw at each other. Well, liquids are like these "ball pits." With very little force, the balls can be moved from one place to another because they don't stick very tightly to surrounding balls.
The molecules in liquids are held together by forces referred to as "intermolecular forces." Let's take a look at how they work.
Dipole-Dipole Forces
Many covalent molecules stick together like little magnets. One side of the molecule has some positive charge on it while another side of the molecule contains some negative charge. Generally, things that have both positive and negative charge on them are referred to as being "polar."
Bad Reactions
Not all molecules that contain polar bonds are necessarily polar molecules. For example, if all the bonds are arranged at equal angles from the center of the atom (as in a Mercedes hood ornament or the points of a pyramid), the charges will be pulled equally in all directions, canceling each other out.
Consider NF3, for example. Using the rule that electronegativity increases as you move from left to right across a row of the periodic table, fluorine is more electronegative than nitrogen, meaning that it pulls on electrons more strongly than nitrogen. Let's examine what this looks like in a single N-F bond:
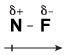
Figure 13.2Because fluorine is more electonegative than nitrogen, it pulls some of the electrons away from it and has a partial negative charge.
Let's learn what the symbols in this figure mean. Fluorine is more electronegative than nitrogen, so it pulls harder on the electrons in the covalent bond than nitrogen does. As a result, the electrons tend to spend more time hanging around the fluorine atom than the nitrogen atom. This uneven sharing of electrons causes fluorine to have a partial negative charge, denoted by the symbol δ-.
Likewise, if fluorine is pulling electrons away from nitrogen, then nitrogen has fewer electrons hanging around it, giving it a partial positive charge, δ+. Because the electrons in this bond are distributed unevenly, it's referred to as being a "polar covalent bond." Polar covalent bonds are formed whenever two elements with dissimilar electronegativities form covalent bonds.
A good way to remember that the dipole arrow points toward negative instead of positive is that the back end looks like an addition sign. This addition sign always points toward the partial positive charge in the molecule, while the head of the arrow points toward the partial negative charge.
Let's examine the structure of the entire NF3 molecule:

Figure 13.3Each fluorine atom pulls electrons away from nitrogen. As a result, the side of the molecule containing the three fluorine atoms gains a partial negative charge, making the whole molecule polar.
Molecular Meanings
Dipole-dipole forces are the attractive forces that hold polar molecules together. This attraction is caused when the partially negative side of one molecule interacts with the partially positive side of another.
As you can see, the three polar N-F bonds are all pointing in the same direction. Because the side of the molecule containing the fluorine atoms has three partial negative charges and the side of the molecule containing the nitrogen atom has a partial positive charge, the whole molecule is polar.
Similarly, we can see that OF2 is a polar molecule because both fluorine atoms contain a partial negative charge:

Figure 13.4Both fluorine atoms pull electrons from oxygen, causing both of the O-F bonds to be polar and the whole OF2 molecule to be polar.
Molecular Meanings
The term hydrogen bond isn't technically accurate. Though the interaction between hydrogen and highly electronegative atoms is strong for an intermolecular force, it's nowhere near as strong as a true chemical bond. However, the term hydrogen bond is very commonly used, and it should be used to describe these interactions.
In a liquid containing polar molecules, the side of the molecule with partial positive charge will align itself with the partially negative side of neighboring molecules. The attractive force between the molecules that results from this interaction is referred to as a dipole-dipole force. An example of how this looks is shown in the following figure.
Chemistrivia
The two strands of a DNA molecule are stuck to each other by hydrogen bonds between each base pair. Hydrogen bonds are much weaker than either ionic or covalent bonds, allowing the two strands in DNA to "unzip" from each other with relative ease.
Dipole-dipole forces, while strong enough to keep the molecules in a liquid together, are much weaker than ionic or covalent bonds. As a result, covalent molecules are able to move freely throughout the liquid.
Hydrogen Bonding
Chemistrivia
London dispersion forces are temporary dipole to disappear. As a result, this strongest between very large molecules because the area of the molecule that can become temporarily polarized is larger.
Some very polar covalent compounds contain a hydrogen atom bonded to a nitrogen, fluo rine, or oxygen atom. As a result, the hydro gen atom on one molecule (which has a high partial positive charge) has a very strong attrac tion to the lone pair electrons on the N, F, or O atom on a neighboring molecule. This very strong force is referred to as a hydrogen bond.
The Mole Says
To recap: Hydrogen bonds are the strongest intermolecular force, dipole-dipole forces are of intermediate strength, and London dispersion forces are weakest. None of these three forces is anywhere near as strong as covalent bonds or the attractions between cations and anions.
To understand why this happens, let's use the example of hydrofluoric acid, HF. In HF, we have a very polar H-F bond due to fluorine's extremely high electronegativity. As a result, most of the electrons are pulled toward fluo rine, leaving very little electron density around hydrogen.

Figure 13.5Polar molecules align themselves to maximize the number of attractions between opposite charges and minimize the number of repulsions between similar charges.

Figure 13.6In HF, fluorine pulls most of the electron density from hydrogen. Because hydrogen has no inner electrons, the partial positive charge on it is very strong, leading to very strong interactions with the lone pairs on the fluorine atoms from other HF molecules.
Because hydrogen has very little electron density, it has a partial positive charge. However, unlike other elements that have a partial positive charge, hydrogen has no inner electrons to shield the nucleus from other atoms. As a result, atoms with partially negative charge have extremely strong electrostatic interactions with hydrogen. These hydrogen bonds, while still not as strong as covalent bonds or the attractive forces between anions and cations, are much stronger than other intermolecular forces.
London Dispersion Forces
So far, we've seen the forces that bind together polar molecules in a liquid. What kind of forces hold the molecules in a nonpolar liquid together? You may be surprised to find that nonpolar molecules also depend on the attraction of opposite charges to stay together in a liquid.
How does this process work? After all, nonpolar molecules, by definition, don't normally have any partial positive or negative charge under normal circumstances! The following figure shows how this works when helium is liquefied:

Figure 13.7London dispersion forces are created when one molecule with a temporary dipole causes another to become temporarily polar.
In the top illustration, we see two helium atoms next to each other. As expected, neither of the atoms has a partial charge. However, if the electrons on one of the helium atoms were to temporarily move to one side of the helium atom, this atom would become very temporarily polar—this is shown in the second illustration. Because the atom on the left is polar, the electrons on the helium atom on the right are attracted toward it, causing the second atom to also become temporarily polar. The attractive force of these two temporary dipoles is referred to as a London dispersion force.
Similarly, these temporarily induced dipoles can take place in nonpolar molecules as well. For example, two methane molecules can undergo the same process and become attracted to each other by London dispersion forces.
As you might expect, this effect is very temporary, because the random movements of electrons within an orbital quickly cause the very weak, very short-lived force is nowhere near as strong an interaction as either dipole-dipole forces or hydrogen bonds.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
