Chemistry: What's a Liquid?
What's a Liquid?
This seems like a silly question because we all have a good feeling for what a liquid is. If I throw something on you and it pokes you in the eye, it's probably not a liquid. On the other hand, if it makes your shirt feel wet and sticky, it probably is.
Liquids are the phase of matter in which molecules can move around freely, but still experience forces that keep them together. For example, if you pour a glass of water on the floor, the water will tend to pool rather than spread into an infinitely thin layer. As a result, we classify water as a liquid under standard conditions.
Molecular Meanings
A liquid is the form of matter in which molecules move around freely but still experience attractive forces.
A general property of liquids is that their volumes remain constant even if their shapes change. For example, you can easily put 500 mL of water into a rubber glove; when you stretch the rubber glove, the shape of the water changes but the volume doesn't.
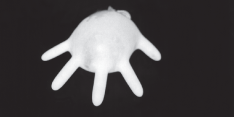
Figure 13.1This rubber glove contains 500 mL of water. No matter what shape it's forced into, the volume of the water remains the same.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
