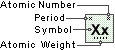Actinium
Symbol
Ac
Atomic Number
89
Atomic Weight
227.0278
Element Classification
Discovered By
Andre-Louis Debierne
Discovery Date
1899 (France)
Name Origin
Greek: akis, aktinos (ray).
Density(g/cc)
n/a
Melting Point
1320
Boiling Point(K)
3470
Appearance
Heavy, Silvery-white metal that is very radioactive
Atomic Radius(pm)
188
Atomic Volume(cc/mol)
22.54
Covalent Radius(pm)
n/a
Ionic Radius
118 (+3e)
Specific Heat
n/a
Fusion Heat
(10.5)
Evaporation Heat(kj/mol)
(292.9)
Thermal Conductivity(@25°C W/m K)
n/a
Debye Temperature
n/a
Pauling Negativity Number
1.1
First Ionizing Energy(kj/mol)
665.5
Oxidation States
3
Electronic Configuration
[Rn] 6d1 7s2
Lattice Structure
Face-Centered Cubic(FCC)
Lattice Constant
5.310
Lattice C/A Ratio
n/a