Chemistry: Factors That Affect Solubility
Factors That Affect Solubility
Sometimes we want a thing to dissolve quickly because we get bored sitting in front of a beaker watching it. Sometimes we want a larger quantity of a solute to dissolve than we could normally achieve. Before this section, both of these things would be impossible. However, now that you understand how solutions work, I feel confident in handing you the following ways of affecting solubility.
Surface Area of the Solute
Let's imagine that you're trying to dissolve 1.0 grams of sodium chloride in a glass of water. Which would dissolve more quickly: a large 1.0 gram crystal or 1.0 grams of salt ground into a fine powder?
If you guessed that the powder would dissolve more quickly, you're right. Because the powder has a larger surface area than the crystal, more of the ions in the salt are exposed to the solvent at any given time, causing them to dissolve more quickly. It should be noted that breaking a solute into smaller pieces doesn't change how much of it will dissolve, it only changes how quickly it will dissolve.
Pressure
When dissolving a gas within a liquid, the pressure of the gas has a huge effect on its solubility. When the pressure of a gas is low, the number of gas molecules that hit the surface of the liquid at any given time is low; as a result, there are fewer chances for the gas to dissolve. However, if the pressure of the gas is increased, the number of collisions between the gas molecules and the solvent increases, which causes more of the gas molecules to dissolve.
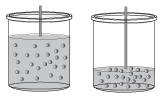
Figure 14.4(a) In the first piston shown, thepressure of the gas is low and the gas molecules don't collide with the solvent very frequently. (b) If the pressure is increased in the piston, the gas molecules will undergo more frequent collisions, leading to higher solubility.
The relationship between pressure and solubility was first expressed in 1801 in Henry's Law:
- P = kC
Chemistrivia
When divers are deep underwater, the pressure of the water increases the amount of gas from the air they breathe that dissolves in their blood. As they rise, the pressure goes down and the gas becomes less soluble, forming small bubbles in the bloodstream. Because gas bubbles accumulate in one's joints, rising too quickly can increase this amount of gas to dangerous levels. The agony caused by this condition causes people to curl up in the fetal position, so it's called "the bends."
In this equation, P represents the pressure of the gas above the solvent, k is a mathematical constant with positive value that depends on the particular solution being studied, and C represents the concentration of the gaseous solute in the solution. As you can see from the equation, the higher the pressure of the gas, the more concentrated the solution will be.
Though pressure is an important factor in the solubility of a gas, pressure has very little effect on the solubilities of liquids or solids.
Temperature
The temperature of a liquid affects the solubility of both solids and gases. Generally, increasing the temperature of a solvent increases the solubility of most ionic compounds (though there are exceptions). More important, increasing the temperature of a solvent usually increases the rate at which a solute dissolves, which is why it's easier to dissolve sugar in hot tea than cold tea. Gases, on the other hand, become less soluble as the temperature of the solution increases, which is why carbonated beverages (which contain CO2) go flat more quickly on hot days than on cold ones.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
