Chemistry: How Ionic Compounds Are Formed
How Ionic Compounds Are Formed
As you might expect from the previous section, the octet rule plays a huge role in ionic compound formation. Let's imagine what happens when lithium reacts with chlorine to form an ionic compound.
Lithium has very low electronegativity, meaning that it tends not to want electrons. Chlorine, on the other hand, has a very high electronegativity, meaning that it wants to gain electrons. As a result, lithium gives electrons to chlorine when one atom of each comes into contact with the other.
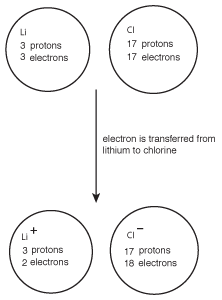
Figure 8.2Chlorine's high electronegativity causes it to pull electrons from lithium, resulting in the formation of the ionic compound LiCl.
When this occurs, lithium goes from having no charge to a +1 charge, while chlorine goes from neutral to having a -1 charge. Because the lithium cation and chlorine anion have opposite charges, they attract one another and form lithium chloride, LiCl.
Generally, ionic compounds are formed whenever two elements with very dissimilar electronegativities (greater than 2.1) bond with each other. As a result, oxygen (electronegativity = 3.4) will form an ionic compound with lithium (electronegativity = 1.0) because the difference in electronegativity between these elements is 3.4 - 1.0 = 2.4. Oxygen, however, does not form ionic compounds with nitrogen (electronegativity = 3.0) because their electronegativities are so similar. Because metals and nonmetals frequently have such dissimilar electronegativities, it's usually a good guess that compounds formed by the combination of metals and nonmetals are ionic.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
