Chemistry: Properties of Covalent Compounds
Properties of Covalent Compounds
When we talked about solid ionic compounds in Ionic Compounds, we found that their properties often derive from the strong attraction of opposite electrical charges. It should not come as a surprise to find that the properties of covalent compounds are largely owing to the nature of covalent bonds.
One of the most important things to remember about covalent compounds is that they're not ionic. This seems obvious, but the difference is actually subtler than you may imagine. To illustrate this concept, take a look at the following figure:
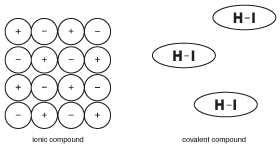
Figure 9.5The properties of solid ionic compounds are based on the fact that many ions are rigidly held in place with electrical forces. Molecules in covalent compounds, however, operate with relative independence from neighboring molecules.
Unlike ionic compounds, where all of the ions in a large crystal help to hold each other together, the molecules in a covalent compound are held together by forces called "intermolecular forces," which are much weaker than chemical bonds (more about intermolecular forces in Solids). As a result, the molecules in a covalent compound are not attracted to each other as much as the ions in ionic compounds. This difference in structure is important in understanding the properties of covalent compounds.
Covalent Compounds Have Low Melting and Boiling Points
As mentioned in Ionic Compounds, a large amount of energy is required to melt an ionic compound because of the strong interactions between the cations and anions in an ionic crystal. However, in covalent compounds, all molecules are bound only weakly to neighboring molecules; therefore, it takes very little energy to separate covalent molecules from one another.
Bad Reactions
Many beginning chemistry students falsely believe that when a covalent molecule melts, covalent bonds are broken. This is false. When ionic compounds melt, the ionic attraction fails. When covalent compounds melt, the molecules simply pull away from each other, leaving the bonds intact.
Covalent Compounds Are Poor Conductors
Ionic compounds are great conductors of electricity when dissolved or melted. As mentioned in Ionic Compounds, this is because ionic compounds have mobile ions that are able to transfer electrical charge from one place to another. They also conduct heat very well because the ions are all right next to each other, making it possible for energy to be transferred efficiently from one place to another.
Covalent compounds, on the other hand, are almost always good insulators of both electricity and heat. Electricity is not able to conduct efficiently through covalent compounds because there are no ions to move the electrical charge. An excellent example of this is in your own house, where the metal in your extension cords is covered with plastic to avoid electrocuting your cat. Heat also doesn't travel well through covalent compounds because the molecules aren't as tightly held to each other as the ions in an ionic compound, making heat transfer less efficient. This is why you use oven mitts to take your cookies out of the oven rather than coating your hands with salt.
Covalent Compounds Sometimes Burn
Molecular Meanings
Organic compounds are covalent compounds that contain carbon. They usually also contain hydrogen. and may contain smaller amounts of other elements such as nitrogen, sulfur, phosphorus, oxygen, or any of the halogens.
Many covalent compounds are flammable and burn readily with the addition of heat. The main group of covalent compounds that are flammable are called organic compounds. Organic compounds burn because they contain carbon and hydrogen, both of which combine nicely with oxygen at high temperatures.
It's important to keep in mind that not all covalent compounds burn—for example, water is a covalent compound and you'll have a very hard time starting a fire with it. However, many more covalent than ionic compounds are flammable.
Flammability is a general property of covalent compounds because a large majority of the known covalent compounds are organic. Since most organic compounds burn, we can safely list this as a property of covalent compounds even though there are many covalent compounds that don't burn.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
