Chemistry: Why Are Solids Solid?
Why Are Solids Solid?
Until you started studying chemistry, you probably took it for granted that it's a bad idea to hit yourself on the head with a rock because solids are hard. Now that your chemical studies have begun, the question that arises is "Why are solids hard in the first place?" To answer this question, we need to examine the nature of crystals.
Some Basic Definitions of Crystals
Molecular Meanings
Solids are the state of matter in which the atoms or molecules are locked rigidly in place by bonds or intermolecular forces.
The atoms in many solids are locked into rigid groups called crystals. The atoms, ions, or molecules in crystals are held together by attractive forces between ions of opposite charge, covalent bonds, and other forces between covalent molecules referred to as intermolecular forces (see Liquids and Intermolecular Forces). Overall, the three-dimensional structure of a crystal is referred to as a crystal lattice.
Molecular Meanings
Crystals are regular arrangements of atoms, ions, or molecules stacked into repeating three-dimensional structures. The smallest unit that can be stacked together to re-create the crystal is referred to as a unit cell.
Because crystals have regular arrangements, we can think of them as being large structures consisting of building blocks that repeat over and over again. The smallest unit of crystals that can be stacked together to re-create the entire crystal is referred to as a unit cell.
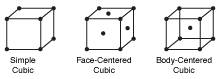
Figure 12.1These three crystals represent three very common cubic crystal structures—simple cubic, face-centered cubic, Cubic and body-centered cubic. The name "cubic" comes from the shape of the unit cell for each crystal.
There are many different types of crystal structures. The type of crystal structure employed by crystalline solids depends on the specific atoms, ions, or molecules present in the crystal. The following figure shows several different types of crystal structures; keep in mind that these are only a sampling of the different types of crystals and are not meant to represent all of the possible types of crystal structures.

Figure 12.2From left to right: the crystal structures of CaTiO3, TiO2, and ZnS.
Close-Packed Crystal Structures
When the atoms in a crystal are all of identical size, they come together in a close-packed structure. As you might guess, the atoms in a crystal are generally only the same size when the atoms are all of the same element. Metals in particular tend to bond in close-packed structures.
Close-packed structures are crystals in which each atom is as close to all its neighboring atoms as possible. If you place a bunch of marbles into a glass, they will arrange themselves so that they take up the least possible space—in short, in a close-packed configuration.
Molecular Meanings
The term close-packed refers to a crystal structure in which all of the atoms are as close together as possible. The two types of close-packed structures are the hexagonal closest-packed (hcp) structure and the cubic closest-packed (ccp) structure.
There are two different ways that atoms can stack together in a close-packed arrangement. The first way is called the hexagonally close-packed (hcp) arrangement, in which the layers of atoms that make up the crystal alternate in an ABAB pattern, with every third layer being directly above the first layer. The second type of arrangement is called the cubic closest-packed (ccp) arrangement. In a ccp arrangement, the layers of atoms are arranged in an ABCABC pattern, allowing every fourth layer to be located directly above the first layer. Both types of packing arrangements are shown in the following figure:

Figure 12.3On the top is the hexagonal close-packed (hcp) arrangement, with its hexagonal unit cell. On the bottom is the cubic closest-packed (ccp) arrangement, with its face-centered cubic (fcc) unit cell.
The atoms in metals usually arrange themselves in either an hcp or ccp arrangement. Generally, the more valence electrons a metal has, the more likely it is to have the ccp structure. Some metals take on body-centered cubic or even simple cubic structures, particularly at high temperatures.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
