Chemistry: Drawing Lewis Structures
Drawing Lewis Structures
Up until this point, we've been determining the types of hybrid orbitals by examining pictures of each molecule being studied. Inevitably, the time has come for you to make some molecular diagrams of your own. These diagrams, called Lewis structures, show all of the valence electrons and atoms in a covalently bonded molecule.
If you've been exposed to Lewis structures before, you may have the erroneous idea that they're difficult to draw. The reason for this is simple: It's a difficult concept for teachers to explain, and books don't usually do much better. Fortunately, I have a foolproof method that can make anybody into a Lewis structure king or queen.
The Mole Says
Lewis structures (named for chemical theorist Gilbert Newton Lewis) are pictures that show all of the valence electrons and atoms in a covalently bonded molecule.
Follow these steps.
Step 1
Count the total number of valence electrons in the molecule.
As an example, let's use carbon tetrachloride, CCl4. The single carbon atom contains four valence electrons, and each of the four chlorine atoms contains seven valence electrons. Therefore, the number of valence electrons for this molecule is 4 + (4 × 7) = 32.
Occasionally, you'll have to find the Lewis structure for a polyatomic ion. To do so, subtract the ionic charge from the valence electron count. For example, NH4+ will have one fewer valence electron that the nitrogen and four hydrogens would have because it has a +1 charge.
Step 2
Count the total octet electron count in the molecule.
You won't find the term "octet electron count" in any textbook because, as far as I know, I made it up. The number of "octet electrons" is equal to the number of valence electrons that each atom will have when they have the same electron configuration as the nearest noble gas (the octet rule). The number of octet electrons that atoms want can usually be determined by the following rules:
- Hydrogen wants two octet electrons.
- Boron wants six octet electrons for neutral molecules and eight for molecules with charge.
- All other atoms want eight octet electrons.
Bad Reactions
If you find that you have a fractional number of bonds, you've made a mistake in an earlier step. Go back to the beginning and check your work!
In our example, carbon wants eight octet electrons and each of the four chlorine atoms also want eight octet electrons. The total number of octet electrons for the molecule will then be equal to 8 + (4 × 8) = 40.
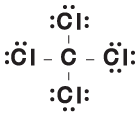
Figure 10.8See, Lewis structures aren't so hard, after all!
Step 3
The Mole Says
If you place single bonds between all of the atoms and there are still some left over, you may need to start putting in double or triple bonds. There's nothing wrong with this—just make sure that all of the atoms have the correct number of bonds when you're done!
Subtract the number of valence electrons from the number of octet electrons to find the number of electrons that are involved in bonding.
In our example, 40 - 32 = 8 bonding electrons.
You've Got Problems
Problem 1: Draw the Lewis structures for the following molecules or polyatomic ions:
(a) NH3
(b) SiO2
(c) OH-1
Step 4
Divide the number of bonding electrons by two to find the number of bonds.
Because there are two shared electrons in every covalent bond, dividing the bonding electrons by two can be used to find the number of chemical bonds. In our example, 8/2 = 4 bonds.
Step 5
Arrange the atoms so the molecule has the same number of covalent bonds that you found in step 4.
In this step, it's tempting to just randomly stick atoms and bonds wherever you can until everything is stuck together. Unfortunately, randomness rarely yields the right answer, so we'll need some rules to help us out.
- The atom that's least abundant in the compound is usually in the middle of the molecule. In our example, we can assume that carbon will probably be in the center of the molecule.
- Hydrogen and the halogens bond once.
- Oxygen's family bonds twice in uncharged molecules and one, two, or three times when present in polyatomic ions.
- Nitrogen's family bonds three times in uncharged molecules and two, three, or four times when present in polyatomic ions.
- Carbon's family usually bonds four times.
- Boron usually bonds three times in uncharged molecules and four times when present in a polyatomic ion.
In our example, CCl4, there are fewer carbon atoms than chlorine atoms, so the carbon atom goes into the middle of the molecule, with four chlorine atoms arranged around it. Between the carbon and each chlorine atom is a single chemical bond, totaling four. In this structure, both carbon and chlorine follow the rules for the number of bonds each wants.
Step 6
Add lone pairs of electrons to each atom until each atom is surrounded by the number of electrons we said they wanted in step 2.
Let's take a look at the carbon atom in our diagram. The four bonds around it contain eight electrons. Since carbon wants eight electrons, it doesn't require lone pairs.
Each chlorine, on the other hand, has only one bond for a total of two electrons. Since chlorine wants eight electrons, three pairs need to be added to each. The completed Lewis structure for CCl4 is shown in the following figure:
Step 7
Check to see if any of the atoms in the molecule have a positive or negative charge.
Add the number of lone pair electrons to the number of bonds for each atom in the molecule. Subtract this number from the number of valence electrons the atom is expected to have (which you already figured out in Step 1) to calculate its charge. Once you've determined the charge on an atom, write it next to the atomic symbol of that atom.
In our example, CCl4, carbon has four bonds around it. Because it normally has four valence electrons, carbon has no charge (4 - 4 = 0). Because each chlorine has one bond and six lone pair electrons (1 + 6 = 7) in this diagram and seven valence electrons, it also has no charge.

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
