Chemistry: Resonance Structures
Resonance Structures
For an additional practice problem, try drawing the Lewis structure for the nitrate ion, NO3-1. This explanation will make a lot more sense if you really draw it, so get a sheet of paper and do it now. I'm going to go get a snack while you work on this.
When you drew the Lewis structure for the nitrate ion, you (hopefully) came up with one of these three structures:
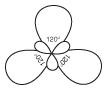
Figure 10.10These represent the three equivalent resonance structures for the nitrate ion.
These three figures are the resonance structures of the nitrate ion. When more than one valid Lewis structure can be drawn for a given arrangement of atoms in a covalent compound, they are referred to as resonance structures. In resonance structures, all of the atoms are located in exactly the same positions, but the numbers and/or locations of the electrons (bonds or lone pairs) may be different.
Molecular Meanings
Resonance structures occur when more than one valid Lewis structure can be drawn for a given arrangement of atoms in a covalent compound. In resonance structures, the atoms are all in the same positions, but the number and locations of bonds and lone pair electrons may be different. The true form of the molecule is an average of the resonance structures that can be written for it.
You may be wondering which of the three equivalent resonance structures is the true structure of the nitrate ion. As it turns out, the actual structure of this ion is an average of the three. Instead of one double bond and two single bonds between the nitrogen and three oxygen atoms, imagine a situation where there's actually 1 1⁄3 bonds between each of the atoms. Likewise, each oxygen atom really has a -1⁄3 charge. Because the concept of odd numbers of bonds and uneven charge is confusing, we usually just draw all of the possible resonance structures for a molecule and let it go at that.
You've Got Problems
Problem 2: Draw both of the possible resonance structures for the formate ion (CHO2-1).

Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.
