DK Science & Technology: Atoms
All matter is made from particles called atoms. The Ancient Greeks described atoms as the smallest particles that make up everything. We now know that atoms are built from even smaller particles. Atoms link together and make MOLECULES.
The radius of a typical atom is one tenth of a billionth of a meter. A string of atoms one meter (about 3 ft) long contains an atom for every person in the world. A cube of sugar contains as many atoms as there are stars in the Universe. The biggest atom (cesium) is nine times the diameter of the smallest atom (helium).
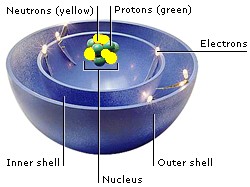
The particles that make up atoms are electrons, protons, and neutrons. Their position in an atom is the ATOMIC STRUCTURE. Electrons are a kind of lepton. Protons and neutrons are made up of three quarks each. Quarks and leptons are fundamental particles—the smallest particles in the Universe.
Protons and neutrons are held together in the nucleus at the center of the atom by a strong force. But this force can be overcome by striking the nucleus with a neutron, a proton, or another particle. The nucleus may split and form new atoms. Atoms are split in this way inside nuclear reactors and during nuclear explosions.
Most of an atom is empty space. Protons and neutrons occupy the nucleus at the center of the atom. Electrons orbit the nucleus like planets around a star. They are grouped in layers called shells.
Electrons carry a negative electric charge, and protons carry a positive charge. The attraction between them holds electrons in orbits. When atoms come together, they share electrons in their outer shells to form chemical bonds.
Different atoms bonded (stuck) together in particular arrangements are called molecules. Water molecules, for example, have two hydrogen atoms bonded to one oxygen atom.